01/04
01/04
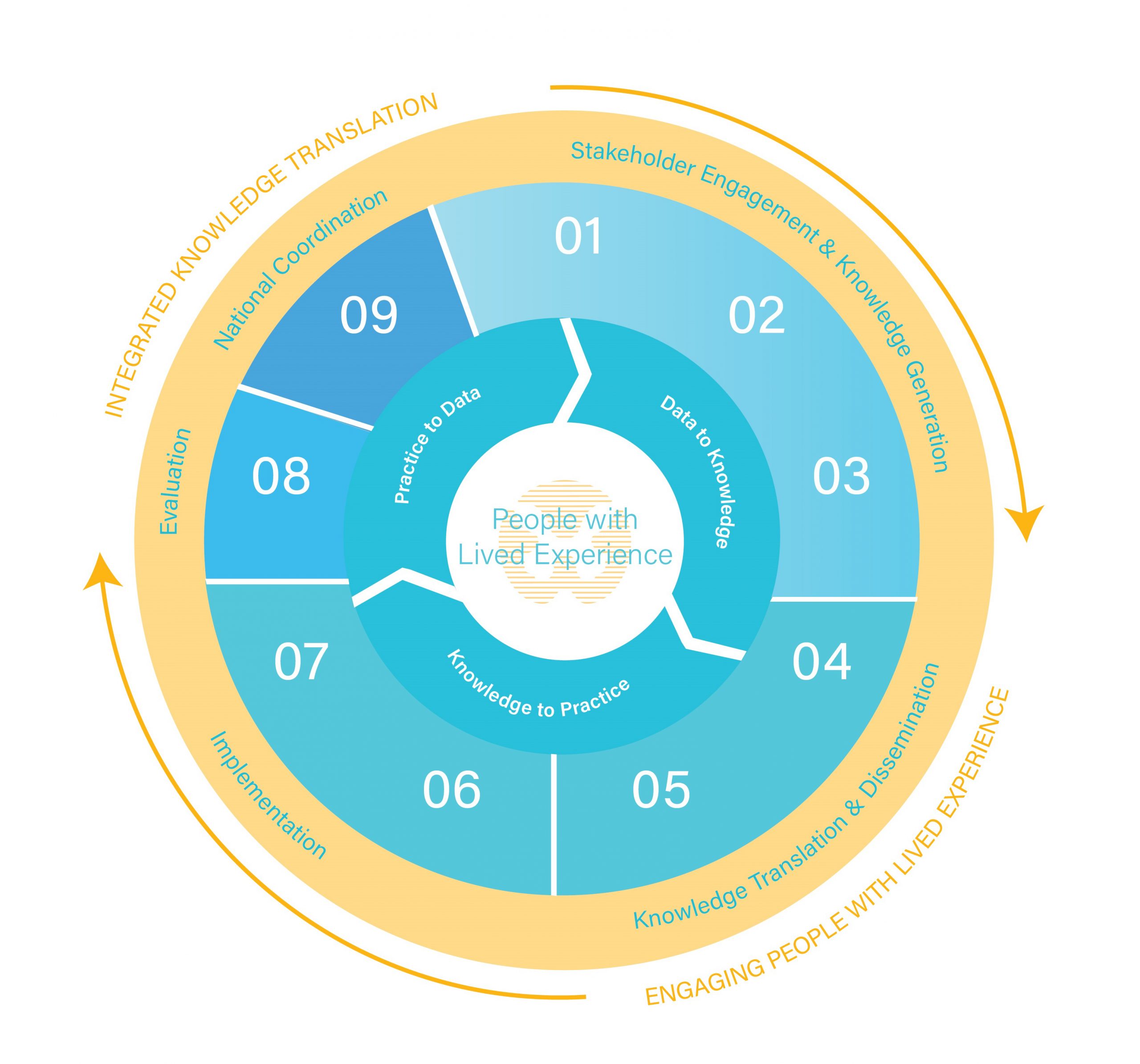
Praxis Data-Knowledge-Practice-Cycle
Applying a learning health systems approach to change the future of spinal cord injury care.
02/04
02/04


Knowledge is Power
Check out SCI-related scientific publications, white papers, conference proceedings, and evaluation reports.
03/04
03/04


Grants & Funding
Discover the latest in grants and funding at Praxis Spinal Cord Institute.
04/04
04/04


How You Can Help
Make a difference in the lives of those living with SCI.


We Are Praxis Spinal Cord Institute
Praxis is a Canadian-based not-for-profit organization that leads global collaboration in spinal cord injury research, innovation and care. We accelerate the translation of discoveries and best practices into improved treatments for people with spinal cord injuries.


Latest News
Keep up-to-date with the latest news and updates from Praxis.




Read the Praxis annual report 2022



Implementing Activity-Based Therapy for Spinal Cord Injury Rehabilitation in Canada: Challenges and Proposed Solutions


A look at Spinal Cord Injury in Canada in 2021



Standing and Walking Assessment Tool (SWAT)Information at practitioner fingertips for best practices in care


Key Initiatives + Resources


Our Impact
At Praxis, we are driven by our vision of a world without paralysis after SCI. Since 2009, we have worked to make exceptional improvements in the health of people with SCI.


Co-Creating a National SCI Care Strategy
How Would You Like To Change The World?
Imagine improving the lives of millions of people around the world who live with a spinal cord injury.
DONATE NOW



Imagine a world without paralysis after spinal cord injury.





